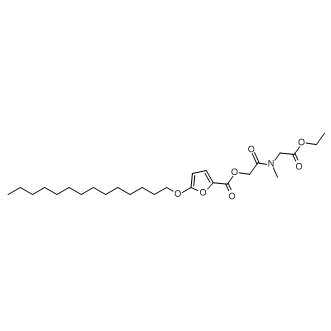
Olumacostat glasaretil
CAS No. 1261491-89-7
Olumacostat glasaretil( Olumacostat glasaretil | DRM01B | DRM-01B )
Catalog No. M11083 CAS No. 1261491-89-7
A small molecule inhibitor of acetyl coenzyme A (CoA) carboxylase (ACC); inhibits de novo lipid synthesis in primary and transformed human sebocytes.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 55 | In Stock |


|
| 5MG | 87 | In Stock |


|
| 10MG | 132 | In Stock |


|
| 25MG | 251 | In Stock |


|
| 50MG | 430 | In Stock |


|
| 100MG | 627 | In Stock |


|
| 200MG | 893 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameOlumacostat glasaretil
-
NoteResearch use only, not for human use.
-
Brief DescriptionA small molecule inhibitor of acetyl coenzyme A (CoA) carboxylase (ACC); inhibits de novo lipid synthesis in primary and transformed human sebocytes.
-
DescriptionA small molecule inhibitor of acetyl coenzyme A (CoA) carboxylase (ACC); inhibits de novo lipid synthesis in primary and transformed human sebocytes; reduces saturated and monounsaturated fatty acyl chains across lipid species, including di- and triacylglycerols, phospholipids, cholesteryl esters, and wax esters; significantly reduces hamster ear sebaceous gland size; a pro-drug of TOFA with enhance delivery in vivo.Other Indication Phase 3 Clinical(In Vitro):Acetyl coenzyme A carboxylase controls the first, rate limiting step in fatty acid biosynthesis. Olumacostat glasaretil inhibits de novo lipid synthesis in primary and transformed human sebocytes. At 3 μM, olumacostat glasaretil reduces fatty acid synthesis to at or below baseline levels. 14C-acetate incorporation levels are 85%-90% lower for SEB-1 cultures treated with olumacostat glasaretil at 20 μM compared to control samples. At 3 μM, olumacostat glasaretil reduces sebocyte triacylglycerol, cholesteryl/wax ester, diacylglycerol, cholesterol and phospholipid levels from control values on average by approximately 86%, 57%, 51%, 39% and 37%, respectively. (In Vivo):Olumacostat glasaretil is a pro-drug of the ACC inhibitor 5-(tetradecyloxy)-2-furoic acid (TOFA) and is designed to enhance delivery in vivo. Topical application of olumacostat glasaretil but not TOFA significantly reduces hamster ear sebaceous gland size. HPLC analyses of hamster ear extracts shows that olumacostat glasaretil treatment increases ACC levels and the ratio of acetyl-CoA to free CoA in tested animals, indicating increased fatty acid oxidation. These changes are consistent with ACC inhibition. Matrix-assisted laser desorption/ionization (MALDI) imaging reveals that OG applied onto Yorkshire pig ears accumulates in sebaceous glands relative to the surrounding dermis. At week 12, OG treatment shows greater reductions from baseline in inflammatory lesions and noninflammatory lesions, and more patients with greater than or equal to 2-grade improvement in investigator global assessment score than vehicle.
-
In VitroAcetyl coenzyme A carboxylase controls the first, rate limiting step in fatty acid biosynthesis. Olumacostat glasaretil inhibits de novo lipid synthesis in primary and transformed human sebocytes. At 3 μM, olumacostat glasaretil reduces fatty acid synthesis to at or below baseline levels. 14C-acetate incorporation levels are 85%-90% lower for SEB-1 cultures treated with olumacostat glasaretil at 20 μM compared to control samples. At 3 μM, olumacostat glasaretil reduces sebocyte triacylglycerol, cholesteryl/wax ester, diacylglycerol, cholesterol and phospholipid levels from control values on average by approximately 86%, 57%, 51%, 39% and 37%, respectively.
-
In VivoOlumacostat glasaretil is a pro-drug of the ACC inhibitor 5-(tetradecyloxy)-2-furoic acid (TOFA) and is designed to enhance delivery in vivo. Topical application of olumacostat glasaretil but not TOFA significantly reduces hamster ear sebaceous gland size. HPLC analyses of hamster ear extracts shows that olumacostat glasaretil treatment increases ACC levels and the ratio of acetyl-CoA to free CoA in tested animals, indicating increased fatty acid oxidation. These changes are consistent with ACC inhibition. Matrix-assisted laser desorption/ionization (MALDI) imaging reveals that OG applied onto Yorkshire pig ears accumulates in sebaceous glands relative to the surrounding dermis. At week 12, OG treatment shows greater reductions from baseline in inflammatory lesions and noninflammatory lesions, and more patients with greater than or equal to 2-grade improvement in investigator global assessment score than vehicle.
-
SynonymsOlumacostat glasaretil | DRM01B | DRM-01B
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaOther Indications
-
IndicationOther Disease
Chemical Information
-
CAS Number1261491-89-7
-
Formula Weight481.6221
-
Molecular FormulaC26H43NO7
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESO=C(C1=CC=C(OCCCCCCCCCCCCCC)O1)OCC(N(CC(OCC)=O)C)=O
-
Chemical Name2-Furancarboxylic acid, 5-(tetradecyloxy)-, 2-[(2-ethoxy-2-oxoethyl)methylamino]-2-oxoethyl ester
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Bissonnette R, et al. J Am Acad Dermatol. 2017 Jan;76(1):33-39.
2. Hunt DW, et al. J Invest Dermatol. 2017 Jul;137(7):1415-1423.
3. Melnik BC. J Invest Dermatol. 2017 Jul;137(7):1405-1408.
molnova catalog



related products
-
Cyclo(Ala-Tyr)
Cyclo(Val-Tyr) is a product from the culture of Pseudomonas putida.
-
Sodium 3-nitrobenzen...
Sodium 3-nitrobenzenesulfonate is a mild oxidant. Sodium 3-nitrobenzenesulfonate can protect the shade when fabrics are printed or steamed in pad dyeing and counteract the effect of reducing substances.
-
11-Oxomogroside IVa
The fruits of Siraitia grosvenorii Swingle.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


